Novel Compact Settlers ...
We have now developed more compact and efficiently scaled-up versions of this proven technology and filed patent applications on four of these novel designs.
Using these more efficient and compact designs, the powerful proven inclined settler technology can now be adapted for therapeutic protein production from recombinant microbial yeast cells as well.
This picture shows a lab/pilot scale cell retention device (at the left) attached to a 5 liter bioreactor growing yeast Pichia pastoris cells continuously in a high cell density perfusion operation for several weeks.
The smaller device shown (at the right) is expected to generate about 5 vvd clarified harvest stream containing only dead cells and cell debris, along with secreted product, from a 5 liter perfusion bioreactor culture of mammalian cells.
Our patent-pending internal settling surfaces make it a very powerful and compact cell separation device, achieving selective removal of dead cells and cell debris via the harvest stream and complete recycle of live and productive cells back to the perfusion bioreactor.

Perfusion Bioreactor culture of yeast cells with a new Compact Cell Settler prototype
We have increased the inclined settler surfaces enormously in the latest design of our novel Compact Cell Settler with the same footprint as a CS-10.
Consequently, we are able to achieve clarified harvest at much higher perfusion flow rates exceeding 5 liters/day from a 5-liter perfusion bioreactor growing yeast P. pastoris cells, crossing the typical perfusion rate of 1 volume exchange per day in mammalian cell cultures.
Shown in Figure, the bioreactor OD600 remains above 700 for over 2 months. Small fluctuations in bioreactor OD from 200 hours to 1000 hours are due to our manipulations of the perfusion rate and feed glycerol concentration. Settler top effluent or harvest is very clarified with its OD600 mostly between 10 and 30, representing less than 5% of the yeast cells in the bioreactor.
This same size settler (12” diameter, shown in bottom photo of previous page) is predicted to be capable of achieving more than 250 liters/day of clarified harvest from mammalian cells, based on the larger size of CHO cells.

Higher Product Harvested from Perfusion Bioreactor compared to Repeated Fed-batch cultures
During the initial fed-batch operation before the perfusion is turned on, the secreted protein product accumulates in the bioreactor to a titer of >1.5 g/L. After perfusion begins, the bioreactor protein titer gets reduced due to its continuous harvest into the effluent and dilution by fresh medium.
The protein titers remain relatively constant from 200 hours to 1000 hours, while the perfusion rate is roughly around 2 liters/day. As the perfusion rate is increased gradually over the two months of perfusion bioreactor operation, particularly after 1000 hours, the protein titers decrease slightly due to the increased harvest and dilution rates.
However, the rate of protein accumulated in the harvest tank (product of perfusion rate and protein titer) increases faster at higher perfusion rates compared to its earlier values at lower perfusion rates. The accumulated protein harvest over the two-month long perfusion operation is 160 g from this 5 liter bioreactor operated at gradually increasing perfusion rates.
This amount is over 4x higher than what can be harvested in the clarified supernatant from about 8 repeated fed-batch culture operations from the same 5 liter bioreactor, during the same two month period.

Compact Cell Settlers work even better for high cell density perfusion bioreactor cultures of mammalian cells
Stainless steel compact cell settlers can be used to achieve high cell densities and high viabilities in continuous perfusion cultures of mammalian (e.g. CHO) cells . In this photo we show a typical attachment of a 6” diameter compact settler to a continuous perfusion bioreactor growing CHO cells for over two weeks. As mammalian cells are > 10 microns, and bigger than the yeast cells, we require a smaller compact settlers than for yeast cells.
Cell culture broth from the bioreactor is pumped with a peristaltic pump into the settler via a horizontal port (hidden behind the stainless steel settler in the foreground with the logo of Sudhin Biopharma Co.). The settled CHO cells are returned from the bottom port via a peristaltic pump into the bioreactor. The flow rate at the settler top port is simply the difference between the two flow rates controlled by the peristaltic pumps: 1. the inlet flow rate from the bioreactor and 2. return flow rate from the settler to the bioreactor.
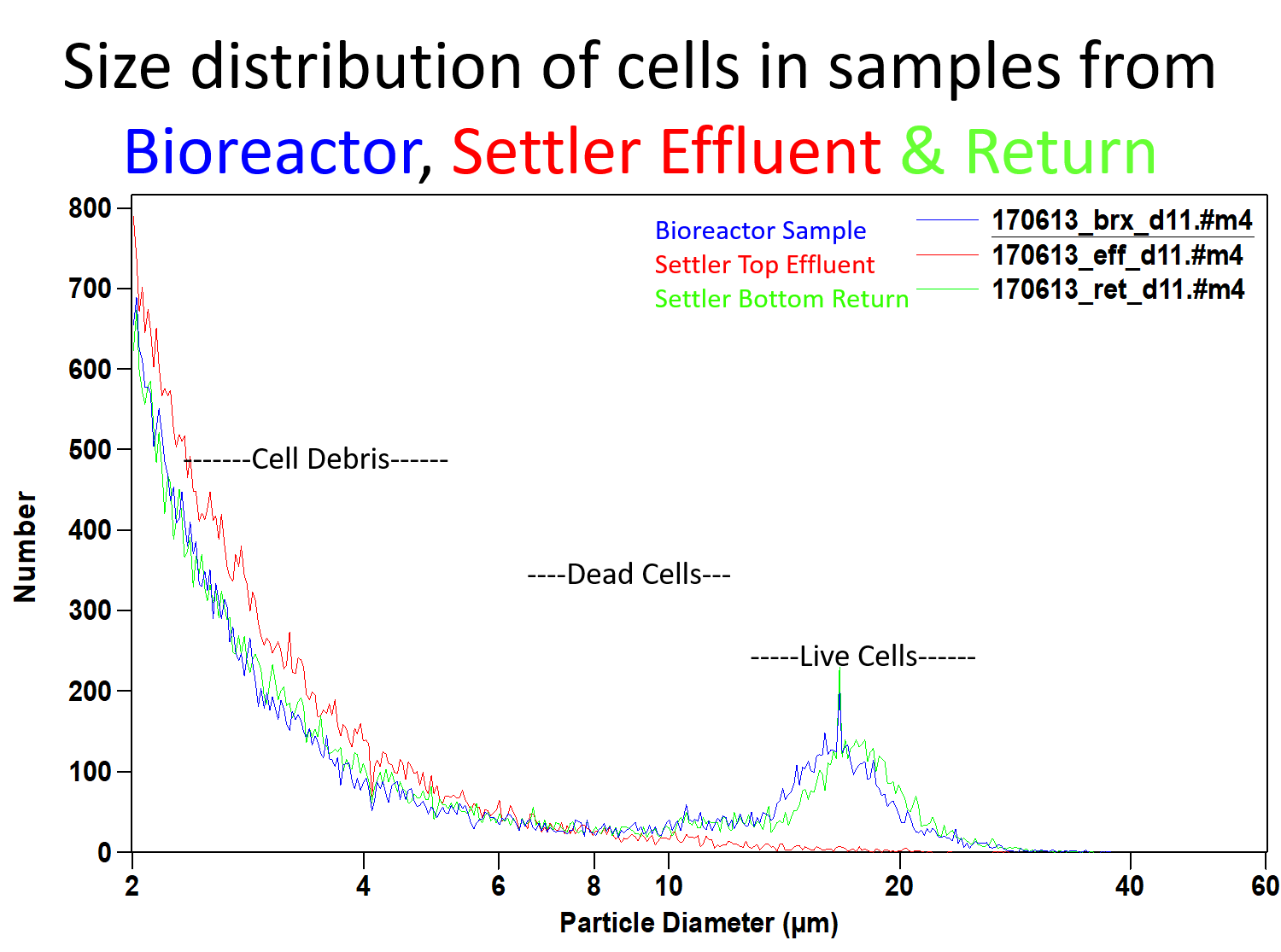
Samples can be taken from the settler top effluent stream and the settler bottom return stream to measure the cell concentrations and viabilities in each stream. We can also measure the size distribution of the cells from the these two samples, as well as those from the bioreactor to analyze the performance of the compact cell settler in selectively removing only the dead cells and cell debris in the settler’s top effluent.

Compact cell settlers remove the smaller dead cells and cell debris selectively in the settler’s top effluent, but not the larger live cells.
In one of our early mammalian cell bioreactor experiments, we had a low cell viability (~50%) in the bioreactor due to a toxic antifoam used. With this low cell viability in the bioreactor, we took a sample and measured the size distribution of cells in the bioreactor sample. We can see an identifiable peak of dead cells in the size range of 8 to 10 microns, and large tail of cell debris in the smaller size range.
When this low viability cells were pumped into the 6” diameter compact cell settler, the settler top effluent contains only those cells which have not settled inside the settler and removed at the bottom port. The sample from the settler top effluent stream shows a larger peak of dead cells in the size range of 8 – 10 microns, and the tail of cell debris in the smaller size range. These data shows very strongly that our compact cell settler can remove only the smaller dead cells selectively and cell debris. More importantly, the top effluent stream contains very few live cells in the size range of 15- 30 microns.
This selective removal of dead cells and cell debris is a unique feature of our compact cell settlers (and our previous inclined lamellar settlers), which is not found with any other total cell retention devices, such as those using membranes, centrifuges, or acoustic resonance trapping methods. Now with our patent-pending compact cell settlers, we provide the same selective removal of dead cells (as the inclined settlers) with a much smaller (1/6th) footprint.

High cell densities are achieved with compact settlers as a selective cell retention device for perfusion bioreactor cultures of mammalian (CHO) cells
In our subsequent experiments with mammalian cells, we have achieved a high cell density of about 20 million cells/ml in the bioreactor within two weeks of bioreactor operation using the compact cell settlers as the selective cell retention device attached to the bioreactor, at a relatively low perfusion flow rate of about 500 ml/day.
The viability percentage of cells in bioreactor remains very high, or increases significantly from a value of about 80% in ten days to about 90 % in two weeks, due to the selective removal of dead cells and cell debris via the settler top effluent stream. The typical drop in viability percentage in fed-page cultures during the first two weeks is monotonic, or can not be reversed at all due to accumulation of dead cells in the fed-batch bioreactor.
The observed increase in viability percentage of our perfusion bioreactor as the culture duration extends into the third week is a unique feature of using settlers (compact or inclined) as a selective cell retention device, but not seen with any other total cell retention devices using membranes, centrifuges or acoustic trapping chambers.

Selective removal of dead cells and cell debris in the settler top effluent and almost complete return of live cells from the settler bottom back to the bioreactor
Size distribution measurements of the three streams, 1. inlet stream from the bioreactor to the settler, 2. settler top effluent / harvest stream and 3. settler bottom return to the bioreactor show reproducibly that (a) the settler top effluent or harvest stream contains only the dead cells and cell debris, but does not contain any live and productive cells and (b) the settler bottom return stream contains almost all the live and productive cells entering from the bioreactor into the settler.
Additional data from trypan blue exclusion assays (not shown here) confirms that the viability of cells in the settler bottom return stream is typically the same as that in the bioreactor and often slightly higher than that in the bioreactor inlet stream. Further the viability of cells leaving in the settler top effluent is very low, confirming that mostly dead cells and cell debris are leaving in the effluent/harvest stream. The number of dead cells and cell debris in the harvest stream are much (several logs) reduced compared to the number of live cells in the bioreactor and in the settler bottom return stream.