Initial Developments ...
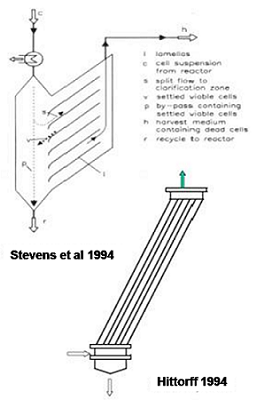
We developed a novel cell retention device, an inclined settler, over twenty years ago, to remove dead mammalian cells selectively and recycle viable and productive cells back to bioreactor.
Selective recycle of live, productive cells to the bioreactor and continuous removal of dead cells results in high cell density and high viability of mammalian cells in continuous perfusion bioreactors, which can therefore be operated for several months at high productivity.
Due to high cell density, viability, production rate, and continuous operations over several months, such perfusion bioreactors produce much more protein than the conventional fed-batch bioreactors of >10x bigger sizes, with plenty of downtime required for cleaning, reseeding, growing, and harvesting activities.

Industrial Scale-Up ...
Inclined settlers have been scaled up (inefficiently) by others and adapted successfully to industrial scale production of complex biological proteins from recombinant mammalian cells by several biotechnology manufacturers.
These innovative companies (Bayer, Biomarin, Eli Lilly, etc.) have utilized this simple and passive technology successfully (as shown above) to operate high cell density continuous perfusion cultures for manufacturing their therapeutic biological molecules.
Other popular cell retention devices, utilizing membrane filtration techniques such as spin filter, tangential flow filtration (TFF) and alternating tangential filtration (ATF), can not separate dead cells from live cells, therefore resulting in membrane clogging and early termination of perfusion culture.
Creative Adaptations ...
Other manufacturers such as Roche have used the inclined settler technology creatively to replace n-1 seed train of batch bioreactors with a single perfusion bioreactor with high viable cell densities (high viability due to removing any dead cells via the inclined settler) to reseed their more traditional fed-batch operations.
Reseeding the large fed-batch production bioreactors with high viability cells from n-1 perfusion bioreactors shortens their initial low cell density growth period and achieves the high cell density production period in much shorter period.
The photo shown on the right is a high resolution version (kindly provided by the authors) of the photo from their publication (Pohlscheidt, M., et al., "Optimizing Capacity Utilization by Large Scale 3000 L Perfusion in Seed Train Bioreactors”, Biotechnology Progress, 29 (1): 222-229, 2013).
